ELECTROPLATING

Electroplating, the process of coating a metal object with a thin layer of another metal by means of electrolysis. Electroplating is used to give metal objects a better appearance or to protect them from corrosion, wear, or rust.
Electroplating is primarily used to change the surface properties of an object (e.g. abrasion and wear resistance, corrosion protection, lubricity, aesthetic qualities, etc.), but may also be used to build up thickness on undersized parts or to form objects by electroforming.
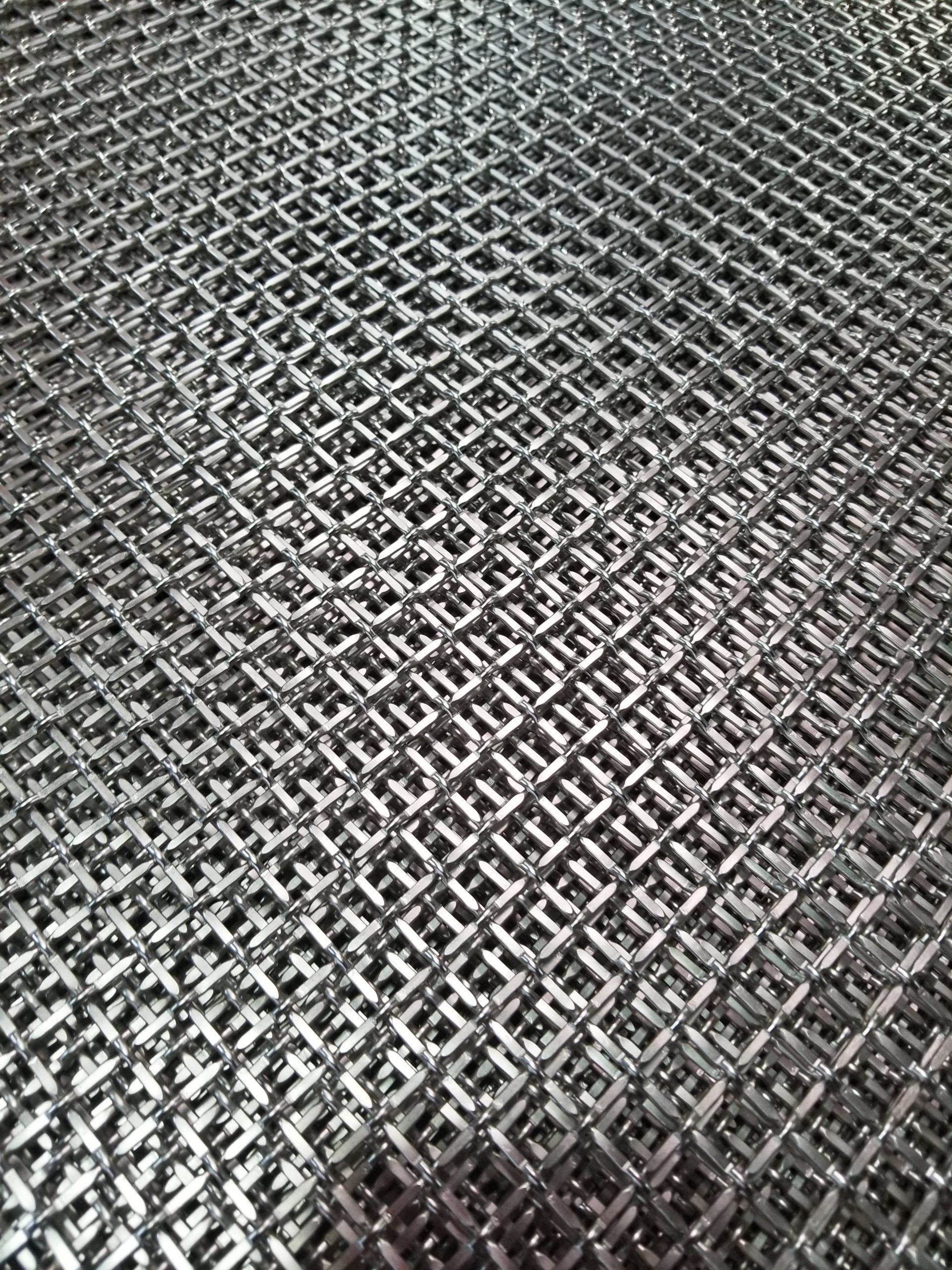
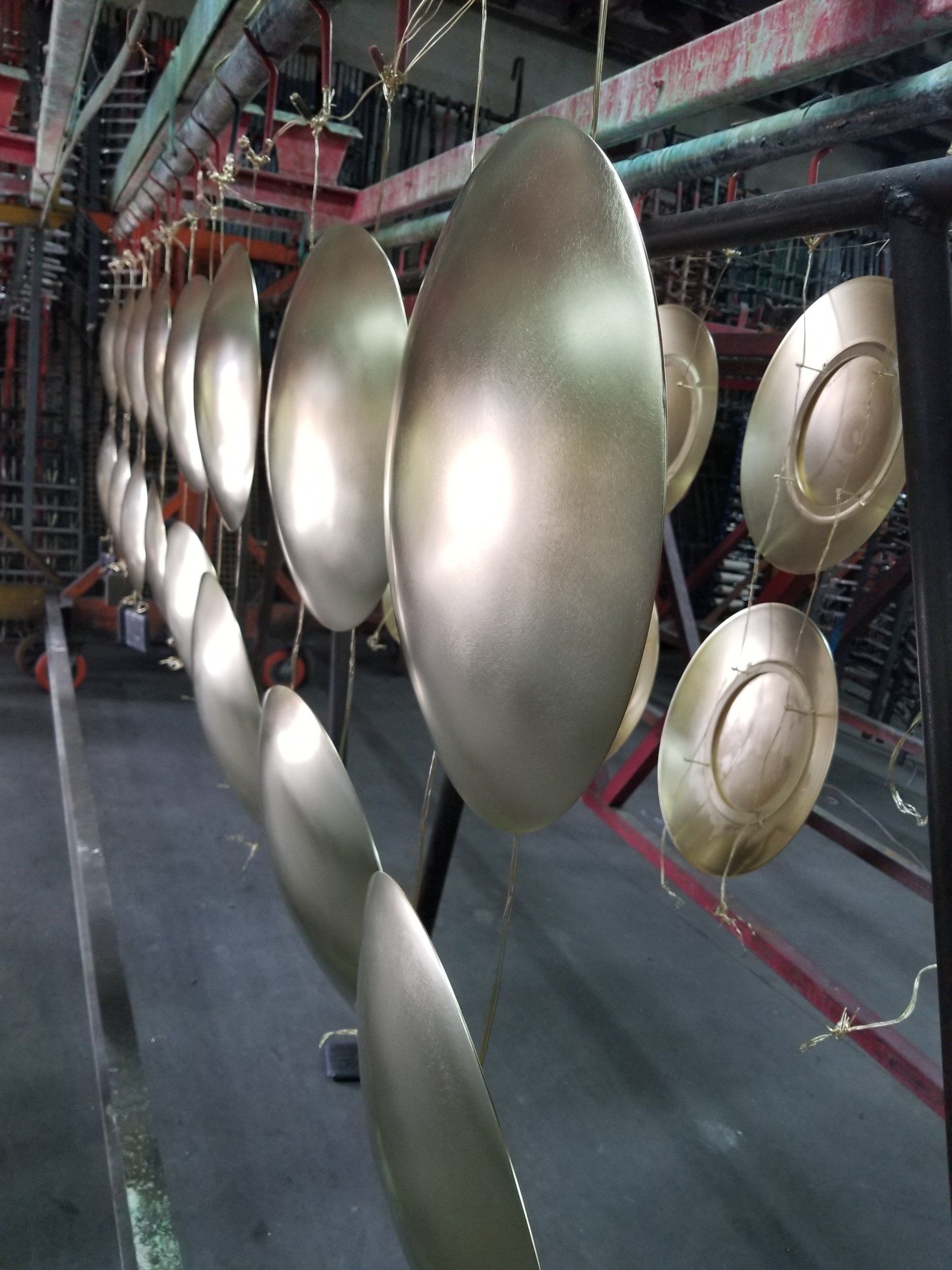
Tableware, trays, decorative pieces, and jewelry are plated with gold or silver to make them more attractive. Copper is coated with chromium to protect it from corrosion. For the same reason iron and steel are plated with nickel, chromium, tin, zinc, or cadmium. Tin cans, for example, are tin-plated steel, and the chrome trim on automobiles is chromium-plated steel. Platinum, palladium, and rhodium are used to coat other metals with a hard, corrosion-resistant surface.
Electroplating, the process of coating a metal object with a thin layer of another metal by means of electrolysis. Electroplating is used to give metal objects a better appearance or to protect them from corrosion, wear, or rust.
Electroplating is primarily used to change the surface properties of an object (e.g. abrasion and wear resistance, corrosion protection, lubricity, aesthetic qualities, etc.), but may also be used to build up thickness on undersized parts or to form objects by electroforming.
Tableware, trays, decorative pieces, and jewelry are plated with gold or silver to make them more attractive. Copper is coated with chromium to protect it from corrosion. For the same reason iron and steel are plated with nickel, chromium, tin, zinc, or cadmium. Tin cans, for example, are tin-plated steel, and the chrome trim on automobiles is chromium-plated steel. Platinum, palladium, and rhodium are used to coat other metals with a hard, corrosion-resistant surface.
The process used in electroplating is called electrodeposition. The most common form of electroplating is used for creating coins such as pennies, which are small zinc plates covered in a layer of copper.
Our Electroplating, Anodizing, Passivation and Coating Services Include:
Zinc, Cadmium, Aluminum Anodizing, Hard Anodizing, Nickel, Electroless Nickel, Tin, Copper, Silver, Gold, RoHS Compliant plating, Passivation, and Coatings (Chromate Conversion Coating, Zinc Phosphate, Manganese Phosphate and Dow 7).

Electroplating, the process of coating a metal object with a thin layer of another metal by means of The process used in electroplating is called electrodeposition. The most common form of electroplating is used for creating coins such as pennies, which are small zinc plates covered in a layer of copper.
Our Electroplating, Anodizing, Passivation and Coating Services Include:
Zinc, Cadmium, Aluminum Anodizing, Hard Anodizing, Nickel, Electroless Nickel, Tin, Copper, Silver, Gold, RoHS Compliant plating, Passivation, and Coatings (Chromate Conversion Coating, Zinc Phosphate, Manganese Phosphate and Dow 7).





 323-268-6353
323-268-6353









 323-268-6353
323-268-6353